- Uv Absorption Water
- Absorption Of Uv-visible To Transmission System
- Absorption Of Uv-visible To Transmission Pressure
Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle is that each compound absorbs or transmits light over a certain range of wavelength. This measurement can also be used to measure the amount of a known chemical substance.
Spectrophotometry is one of the most useful methods of quantitative analysis in various fields such as chemistry, physics, biochemistry, material and chemical engineering and clinical applications. IntroductionEvery chemical compound absorbs, transmits, or reflects light (electromagnetic radiation) over a certain range of wavelength. Spectrophotometry is a measurement of how much a chemical substance absorbs or transmits. Spectrophotometry is widely used for quantitative analysis in various areas (e.g., chemistry, physics, biology, biochemistry, material and chemical engineering, clinical applications, industrial applications, etc). Any application that deals with chemical substances or materials can use this technique. In biochemistry, for example, it is used to determine enzyme-catalyzed reactions. In clinical applications, it is used to examine blood or tissues for clinical diagnosis.
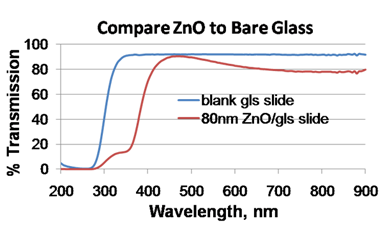

There are also several variations of the spectrophotometry such as atomic absorption spectrophotometry and atomic emission spectrophotometry.A spectrophotometer is an instrument that measures the amount of photons (the intensity of light) absorbed after it passes through sample solution. With the spectrophotometer, the amount of a known chemical substance (concentrations) can also be determined by measuring the intensity of light detected. Depending on the range of wavelength of light source, it can be classified into two different types:. UV-visible spectrophotometer: uses light over the ultraviolet range (185 - 400 nm) and visible range (400 - 700 nm) of electromagnetic radiation spectrum. IR spectrophotometer: uses light over the infrared range (700 - 15000 nm) of electromagnetic radiation spectrum.In visible spectrophotometry, the absorption or the transmission of a certain substance can be determined by the observed color.
Uv Absorption Water
For instance, a solution sample that absorbs light over all visible ranges (i.e., transmits none of visible wavelengths) appears black in theory. On the other hand, if all visible wavelengths are transmitted (i.e., absorbs nothing), the solution sample appears white. If a solution sample absorbs red light (700 nm), it appears green because green is the complementary color of red. Visible spectrophotometers, in practice, use a prism to narrow down a certain range of wavelength (to filter out other wavelengths) so that the particular beam of light is passed through a solution sample. References. Atkins, Peter and Julio de Paula.
Waiting 4 more months for the sake of saving $10-15 dollars is kind of meh, and who knows, at that point, you might want to play Civ 6. Originally posted by High Functioning Sociopath:Exactly what the title says, you guys probably won't know, but when it is can somebody reply to this thread and tell me?Thanks.Id imagine, in the least, around the time Civ 6 gets released, but could actually just drop in price permanently.There is however steam summer sale to consider too, which is around June 23.If it isnt one of the titles to go on sale after summer sale, Id recommend just buying it.  Civ V complete is definitely worth it.
Civ V complete is definitely worth it.
Absorption Of Uv-visible To Transmission System
Physical Chemistry for the Life Sciences. New York: Oxford University Press, 2006. Chang, Raymond. Physical Chemistry for the Biosciences. USA: University Science Books, 2005. Gore, Michael. Spectrophotometry & Spectrofluorimetry.

Absorption Of Uv-visible To Transmission Pressure
New York: Oxford University Press, 2000. Price, Nicholas and Dwek, Raymond and Wormald, Mark. Principles and Problems in Physical Chemistry for Biochemists. New York: Oxford University Press, 1997. Irwin H.
Segel, Biochemical Calculations (How to Solve Mathematical Problems in General Biochemistry), 2nd edition, John Wiley & Sons, 1975. The LibreTexts libraries are and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Unless otherwise noted, LibreTexts content is licensed. Have questions or comments? For more information contact us at or check out our status page at.